
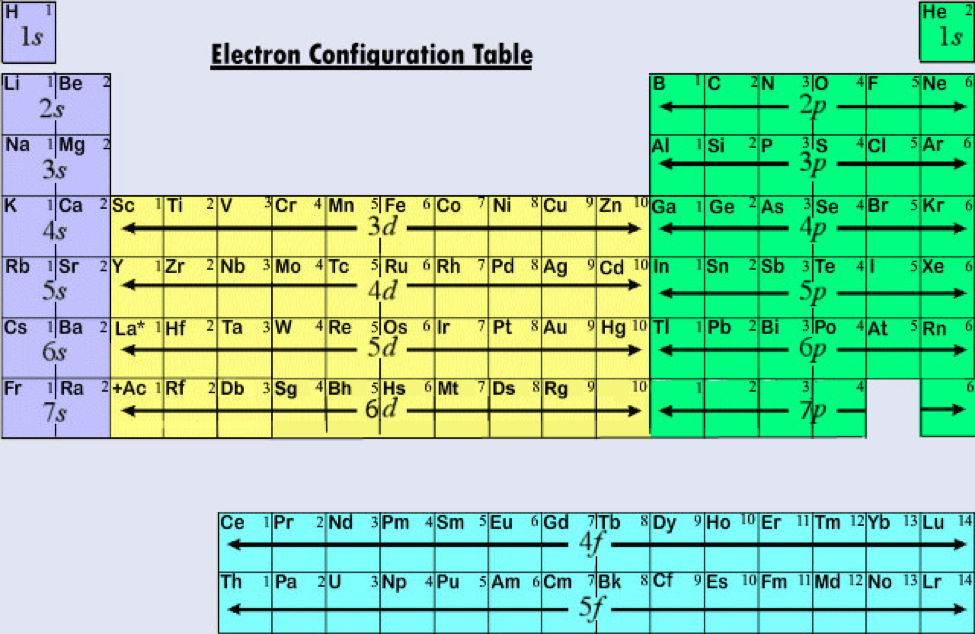
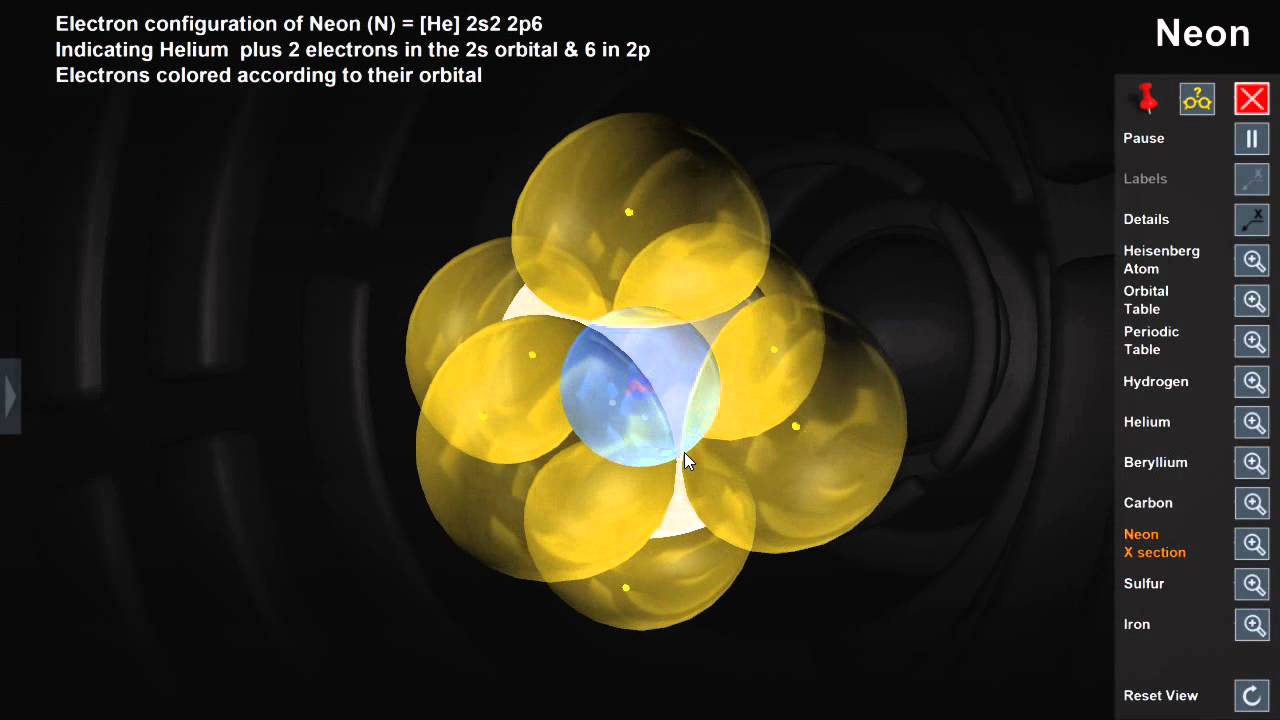
The nucleaus is kind of where the knots are and the electrons can be found either here or here and it look likes a dumbbell shape. Okay, p orbitals look slightly different, this is what a p orbital looks like this is one even though there are two balloons this is one p orbital. So we're going to say 1 and then n equals 2 principal energy level there's an s also with a bigger sphere and there's also p orbitals. So there is one orbital for every s in s orbitals there's only one looks like this. If we take this balloon it's a spherical balloon s orbitals are spherical in shape in the middle we have a nucleus and surrounding it we have the idea of where the electron can be found. We are going to have, we have one orbital, we're going to call it the s orbital and let's give an idea of what the s orbital actually looks like. So in the principal of energy level n=1 n is basically which principal energy level is which. So if you have a nucleus in the middle, here is the nucleus and back here is where the electrons are located, we are going to, there are different energy levels the principal energy levels where electron can be found. So if we go over here, we're going to actually try and describe where an electronic can be located within an atom. And he along with Schrodinger which is he kind of like calculated this huge mathematical idea where electronic can be found came up with this new idea what an atom looks like. So Schrodinger along with Heisenberg came up with a new idea what an atom looks like it's the one we used today Heisenberg uncertainty principal states that it is impossible to know precisely both the velocity and location of an electron at the same time. So we're going to now use the more updated model which is the quantum chemical model. We're not going to use that model anymore, it's extremely outdated we're going to ignore it, it's great for simplistic ideas of how atoms behaves but now we're going to talk about, we're getting more to Chemistry we're going to talk about more in depthy. So we ideally had the idea that atomic, an atom looks like and the nucleus in the middle positively charged nucleus with the electrons orbiting around it. Alright let's talk about the atomic orbitals.


 0 kommentar(er)
0 kommentar(er)
